Reductive deconstruction of organosolv lignin catalyzed by zeolite supported nickel nanoparticles†
Abstract
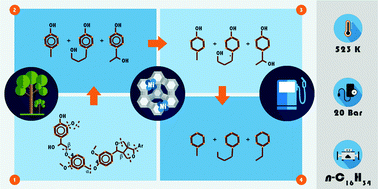
Mechanistic aspects of deconstruction and hydrodeoxygenation of organosolv lignin using zeolite (HZSM-5 and HBEA) and SiO2 supported Ni catalysts are reported. Lignin was deconstructed and converted to substituted alicyclic and aromatic hydrocarbons with 5 to 14 carbon atoms. Full conversion with total yield of 70 ± 5 wt% hydrocarbons was achieved at 593 K and 20 bar H2. The organosolv lignin used consists of seven to eight monolignol subunits and has an average molecular weight of ca. 1200 g mol−1. The monolignols were mainly guaiacyl, syringyl and phenylcoumaran, randomly interconnected through β-O-4, 4-O-5, β-1, 5-5′ and β–β ether bonds. In situ IR spectroscopy was used to follow the changes in lignin constituents during reaction. The reductive catalytic deconstruction of organosolv lignin starts with the hydrogenolysis of aryl alkyl ether bonds, followed by hydrogenation of the aromatic rings to cyclic alcohols. Oxygen is removed from the alcohols via dehydration on Brønsted acid sites to cyclic alkenes that are further hydrogenated.
- This article is part of the themed collection: Lignin chemistry and valorisation

 Please wait while we load your content...
Please wait while we load your content...