Lactic acid production from hydroxyacetone on dual metal/base heterogeneous catalytic systems
Abstract
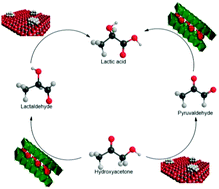
Oxidative aqueous-phase catalytic conversion of hydroxyacetone to lactic acid was investigated under mild and green reaction conditions. Dual metal/base catalyst systems were applied to replace the alkali hydroxide and address the separation requirements and corrosiveness issues usually involved in similar processes. Pt/Al2O3 was taken as the supported metal catalyst while Mg–Al layered double hydroxides (LDH) and their derived 3D mixed oxides were exploited as basic solid catalysts. The anion embedded within the LDH interlamellar domain (nitrate or hydroxyl) and the LDH topology (lamellar or exfoliated 2D nanosheets) were also examined. Activity and chemoselectivity of the dual system were shown to be tailored by the morphology, topology and basicity of the base catalyst. Catalytic activity was up to five times higher by adding a heterogeneous base solid to the metal catalyst. Surface site distribution was also shown to be quite decisive to the catalyst performance; the proximity of metal and basic centres on the same surface rendered highly selective bifunctional catalysts. A remarkable 100% selectivity to lactic acid was reached and the undesired cascade oxidation of lactic to pyruvic acid was hindered.

 Please wait while we load your content...
Please wait while we load your content...