Facile synthesis of new, highly efficient SnO2/carbon nitride composite photocatalysts for the hydrogen evolution reaction†
Abstract
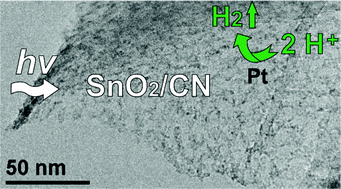
Novel SnO2/carbon nitride photocatalysts having surface areas up to 220 m2 g−1 were prepared for the first time by condensation of dicyandiamide in alkali metal chloride/SnCl2-containing salt melts at 550 °C, without the use of hard templates. XRD and HR-TEM investigations showed that the obtained materials are composed of 5–10 nm SnO2 nanoparticles deposited onto nanosheets set up from 1D-melon ribbons. The morphology and crystalline structure of products appear to be greatly dependent on the synthesis temperature. SnO2/carbon nitride composites are found to be highly efficient in the photocatalytic reactions, as exemplified by Rhodamine B degradation and water reduction using Pt as a co-catalyst. Under the optimized synthesis conditions, these composite photocatalysts achieve hydrogen evolution rates more than 2 times higher than the mesoporous carbon nitride (mp-CN) under visible light irradiation. In principle, this new method based on utilization of MCl/SnCl2 salt melts as a reaction medium allows carrying out various polymerization reactions in the presence of the mild Lewis acid in the solution phase in the wide temperature range of 180–550 °C. Moreover, SnCl2 eutectics are even suitable for post-synthesis modification of the bulk carbon nitride to tune its morphology and greatly increase the surface area and photocatalytic activity.

 Please wait while we load your content...
Please wait while we load your content...