Reaction of isatins with 6-amino uracils and isoxazoles: isatin ring-opening vs. annulations and regioselective synthesis of isoxazole fused quinoline scaffolds in water†
Abstract
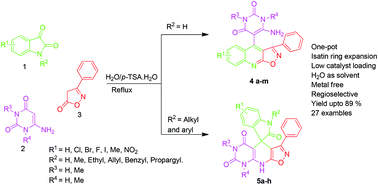
A green and efficient straightforward approach for the regioselective synthesis of novel 6-amino-5-(3-phenylisoxazolo[5,4-b]quinolin-4-yl)pyrimidine-2,4(1H,3H)-diones and 3-methyl-1-phenyl-4-(3-phenylisoxazolo[5,4-b]quinolin-4-yl)-1H-pyrazol-5-amines via the cleavage of the isatin C–N bond followed by a ring expansion reaction in a one-pot manner has been established using environmentally benevolent p-toluene sulphonic acid as a catalyst. An exciting feature of this article is the reaction mechanism that depends on the nature of the group attached to the isatin ring nitrogen atom. The main advantages of this protocol include a short reaction time, an excellent yield, easy work-up, practical simplicity, high regioselectivity and the absence of extraction and chromatographic purification steps.

 Please wait while we load your content...
Please wait while we load your content...