Rapid, energy-efficient synthesis of the layered carbide, Al4C3†
Abstract
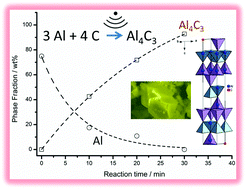
The phase-pure binary aluminium carbide, Al4C3 can be synthesised in vacuo from the elements in 30 minutes via microwave heating in a multimode cavity reactor. The success of the reaction is dependent on the use of finely divided aluminium and graphite starting materials, both of which couple effectively to the microwave field. The yellow-brown powder product was characterised by powder X-ray diffraction, scanning electron microscopy/energy dispersive X-ray spectroscopy thermogravimetric-differential thermal analysis and Raman spectroscopy. Powders were composed of hexagonal single crystallites tens of microns in diameter (rhombohedral space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m; Z = 3; a = 3.33813(5) Å, c = 25.0021(4) Å) and were stable to 1000 °C in air, argon and nitrogen. Equivalent microwave reactions of the elements in air led to the formation of the oxycarbide phases Al2OC and Al4O4C.
m; Z = 3; a = 3.33813(5) Å, c = 25.0021(4) Å) and were stable to 1000 °C in air, argon and nitrogen. Equivalent microwave reactions of the elements in air led to the formation of the oxycarbide phases Al2OC and Al4O4C.

 Please wait while we load your content...
Please wait while we load your content...