Controllable synthesis of nucleotide complex based on pH control: a small-molecule fluorescent probe as an auxiliary ligand to indicate the pre-organization of the nucleotide complex in solution†
Abstract
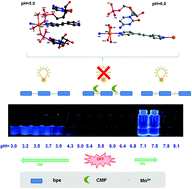
Different CMP–bpe–M(II) (CMP = cytidine monophosphate, bpe = bis(4-pyridyl)ethylene, M(II) = Mn2+ and Co2+) complexes are synthesized controllably based on pH control and well studied by X-ray single-crystal diffraction analysis. Interestingly, the suitable pH conditions are explored by considering the pre-organization modes of CMP, bpe, and M2+ in aqueous solution by fluorescence spectroscopy based on acid/basic titration. The organic base bpe serves as a small-molecule fluorescent probe to indicate the changes of pre-organization modes along with the changes of the solution acidity. Furthermore, a perfect self-complementary sugar–base hydrogen bond is first reported based on the crystal structure analysis in this work, and different chiralities and SHG activities of CMP–bpe–Mn(II) complexes obtained at different pH values are studied.

 Please wait while we load your content...
Please wait while we load your content...