Alkylfluorenyl substituted N-heterocyclic carbenes in copper(i) catalysed hydrosilylation of aldehydes and ketones†
Abstract
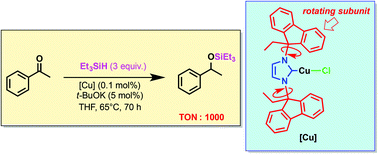
Copper(I) complexes featuring N-heterocyclic carbenes (NHCs) in which the nitrogen atoms are substituted by a 9-ethyl-9-fluorenyl group (EF) have been synthesised and tested in the hydrosylilation of functionalized and/or sterically demanding ketones and aldehydes. These reactions, carried out with triethylsilane as hydride source, were best achieved with the imidazolylidene copper complex 2d in which the EF substituents can freely rotate about the corresponding N–CEF bonds. The remarkable stability of the active species, which surpasses that of previously reported Cu–NHC catalysts is likely to rely on the ability of the NHC side arms to protect the copper centre during the catalytic cycle by forming sandwich-like intermediates, but also on its steric flexibility facilitating approach of encumbered substrates. TONs up to 1000 were reached.

 Please wait while we load your content...
Please wait while we load your content...