Stereoselective formation and catalytic activity of hydrido(acylphosphane)(chlorido)(pyrazole)rhodium(iii) complexes. Experimental and DFT studies†
Abstract
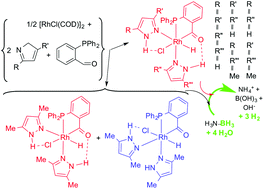
The reaction of [{RhCl(COD)}2] (COD = 1,5-cyclooctadiene) with L = pyrazole (Hpz), 3(5)-methylpyrazole (Hmpz) or 3,5-dimethylpyrazole (Hdmpz) and PPh2(o-C6H4CHO) (Rh : L : P = 1 : 2 : 1) gives hydridoacyl complexes [RhHCl{PPh2(o-C6H4CO)}(L)2] (1). Stereoselective formation of 1-Hpz and 1-Hmpz with pyrazoles trans to hydrido and phosphorus and hydrogen bond formation with O-acyl and chlorido occur. 1-Hmpz is a mixture of two linkage isomers in a 9 : 1 ratio, with two 5-methylpyrazole ligands or with one 3- and one 5-methylpyrazole ligand, respectively. Fluxional 1-Hdmpz undergoes metallotropic tautomerization and is a mixture of equal amounts of 1a-Hdmpz and 1b-Hdmpz, with hydrido trans to pyrazole or chlorido, respectively. Complexes 1 readily exchange hydrido by chlorido to afford [RhCl2{PPh2(o-C6H4CO)}(L)2] (2-Hpz, 2-Hmpz and 2-Hdmpz) as single isomers with cis chloridos and two N–H⋯Cl hydrogen bonds. The reaction of 1 with PPh3 or PPh2OH affords static [RhHCl{PPh2(o-C6H4CO)}(PPh3)L] (3) or [RhHCl{PPh2(o-C6H4CO)}(PPh2OH)L] (4) respectively with trans P-atoms and pyrazoles forming N–H⋯Cl hydrogen bonds. 3-Hpz and 3-Hmpz contain single species with hydrido cis to chlorido, while 3-Hdmpz is a mixture of equal amounts of 3a-Hdmpz and 3b-Hdmpz. Complexes 4, with an additional O–H⋯O hydrogen bond, selectively contain only the cis-H,Cl species with all the three ligands. The reaction of [{RhCl(COD)}2] with L and PPh2(o-C6H4CHO) (Rh : L : P = 1 : 1 : 2) led to complexes with trans P-atoms, [RhHCl{PPh2(o-C6H4CO)}{PPh2(o-C6H4CHO)-κP}L] (5-Hpz, 5a-Hdmpz and 5b-Hdmpz), at room temperature, and to [RhCl{PPh2(o-C6H4CO)}{PPh2(o-C6H4CHOH)}(Hmpz)] (6-Hmpz) or [RhCl{PPh2(o-C6H4CO)}2L] (7) with hydrogen evolution in refluxing benzene. DFT calculations were used to predict the correct isomers, their ratios and the particular intramolecular hydrogen bonds in these complexes. Single crystal X-ray diffraction analysis was performed on 2-Hpz, 3a-Hdmpz and 7-Hpz. Complexes 1 are efficient homogeneous catalysts (0.5 mol% loading) in the hydrolysis of amine– or ammonia–borane (AB) to generate up to 3 equivalents of hydrogen in the presence of air.

 Please wait while we load your content...
Please wait while we load your content...