Towards dipyrrins: oxidation and metalation of acyclic and macrocyclic Schiff-base dipyrromethanes†
Abstract
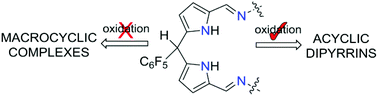
Oxidation of acyclic Schiff-base dipyrromethanes cleanly results in dipyrrins, whereas the macrocyclic ‘Pacman’ analogues either decompose or form new dinuclear copper(II) complexes that are inert to ligand oxidation; the unhindered hydrogen substituent at the meso-carbon allows new structural motifs to form.

 Please wait while we load your content...
Please wait while we load your content...