Electronic structure of β-RbSm(MoO4)2 and chemical bonding in molybdates†
Abstract
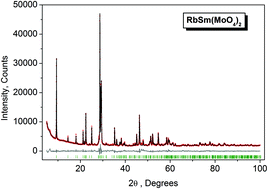
Microcrystals of orthorhombic rubidium samarium molybdate, β-RbSm(MoO4)2, have been fabricated by solid state synthesis at T = 450 °C, 70 h, and at T = 600 °C, 150 h. The crystal structure has been refined by the Rietveld method in space group Pbcn with cell parameters a = 5.0984(2), b = 18.9742(6) and c = 8.0449(3) Å (RB = 1.72%). Thermal properties of β-RbSm(MoO4)2 were traced by DSC over the temperature range of T = 20–965 °C, and the earlier reported β ↔ α phase transition at T ∼ 860–910 °C was not verified. The electronic structure of β-RbSm(MoO4)2 was studied by employing theoretical calculations and X-ray photoelectron spectroscopy. It has been established that the O 2p-like states contribute mainly to the upper part of the valence band and occupy the valence band maximum, whereas the Mo 4d-like states contribute mainly to the lower part of the valence band. Chemical bonding effects have been analysed from the element core level binding energy data. In addition, it was found that the luminescence spectrum of β-RbSm(MoO4)2 is rather peculiar among the Sm3+ containing materials. The optical refractive index dispersion in β-RbSm(MoO4)2 was also predicted by the first-principles calculations.

 Please wait while we load your content...
Please wait while we load your content...