The kinetics and mechanism of photo-assisted Ag(i)-catalysed water oxidation with S2O82−†
Abstract
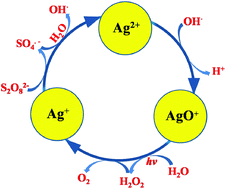
The kinetics of photo-assisted Ag(I)-catalysed water oxidation into O2 with S2O82− has been investigated. When the concentration of Ag+ is less than 7.06 × 10−3 mol L−1, O2-evolution under visible light illumination (λ ≥ 400 nm) obeys the first-order rate law with respect to the concentrations of Ag+ and S2O82−, respectively. The rate law is expressed as −dc(S2O82−)/dt = 2dc(O2)/dt = kLc(S2O82−)c(Ag+), where kL is 12.4 ± 1 mol−1 L h−1 at 24.5 °C and the activation energy is 3.7 × 104 J mol−1. It is found that visible light can improve the evolution of O2 remarkably. Compared with those without illumination, the rate constants under visible light are increased by ca. 3.8 mol−1 L h−1 at 4.5, 11.5, 17.5 and 24.5 °C, which are hardly affected by the reaction temperature. Employing MS/MS, ESR, XRD and UV-visible spectroscopy, the intermediate species {AgS2O8}−, Ag2+, OH˙, Ag2O3 and AgO+ in the process of water oxidation have been detected. Based on the experimental evidence, the mechanism of Ag(I)-catalysed water oxidation with S2O82− has been developed, in which the reaction (AgO+ + H2O → Ag+ + H2O2) is considered as the rate-determining step. The increase of the O2-evolution rate under visible light illumination results from the absorbance of the AgO+ species at 375 nm, promoting the rate-determining step.

 Please wait while we load your content...
Please wait while we load your content...