A density functional theory study on ethylene formation and conversion over P modified ZSM-5
Abstract
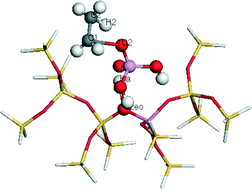
In this work, density functional theory (DFT) was used to choose the suitable ZSM-5 model. The effect of P modification of ZSM-5 on the rate-limiting step of the stepwise reaction pathway of ethylene dimerization, i.e., ethylene protonation, was studied. Note that 14T was used to conduct the transition state search. On the extra-framework modified H3PO4/HZSM-5 zeolite, the adsorption energy of ethylene decreases, which makes products desorb quickly after formation and reduces the incidence of dimerization or polymerization. The ethylene polymerization reaction is the main cause of coking deactivation of ZSM-5. Ethylene protonation reaction is the rate-limiting step of ethylene dimerization, suggesting that after P modification, the activation energy of ethylene dimerization increases to inhibit ethylene dimerization, preventing ethylene polymerization and thus improving the resistance to the coke deposition of ZSM-5, and prolong the life of zeolites. Based on the study results, the inhibition mechanism of P modification to ethylene dimerization was explored to provide a beneficial theoretical guide for designing and improving the catalyst of ethanol dehydration to ethylene.

 Please wait while we load your content...
Please wait while we load your content...