Interfacial zippering-up of coiled-coil protein filaments†
Abstract
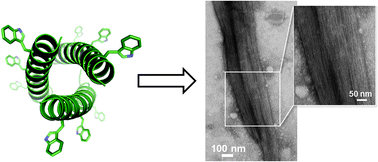
Protein self-assembled materials find increasing use in medicine and nanotechnology. A challenge remains in our ability to tailor such materials at a given length scale. Here we report a de novo self-assembly topology which enables the engineering of filamentous protein nanostructures under morphological control. The rationale is exemplified by a ubiquitous self-assembly motif – an α-helical coiled-coil stagger. The stagger incorporates regularly spaced interfacial tryptophan residues, which allows it to zipper up into discrete filaments that bundle together without thickening by maturation. Using a combination of spectroscopy, microscopy, X-ray small-angle scattering and fibre diffraction methods we show that the precise positioning of tryptophan residues at the primary and secondary structure levels defines the extent of coiled-coil packing in resultant filaments. Applicable to other self-assembling systems, the rationale holds promise for the construction of advanced protein-based architectures and materials.

 Please wait while we load your content...
Please wait while we load your content...