Quantum dynamics of the photostability of pyrazine†
Abstract
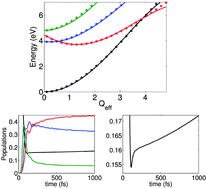
We investigate the radiationless decay of photoexcited pyrazine to its ground electronic state using multireference electronic structure and quantum dynamics calculations. We construct a quadratic vibronic coupling Hamiltonian, including the four lowest electronic states and ten vibrational modes, by fitting to more than 5000 ab initio points. We then use this model to simulate the non-adiabatic excited state dynamics of the molecule using the multi-configuration time-dependent Hartree method. On the basis of these calculations, we propose a new mechanism for this decay process involving a conical intersection between the Au(nπ*) state and the ground state. After excitation to the B2u(ππ*) state, the molecule decays to both the B3u(nπ*) and Au(nπ*) states on an ultrashort timescale of approximately 20 fs. The radiationless decay to the ground state then occurs from the Au(nπ*) state on a much longer timescale.

 Please wait while we load your content...
Please wait while we load your content...