Influence of halide precursor type and its composition on the electronic properties of vacuum deposited perovskite films†
Abstract
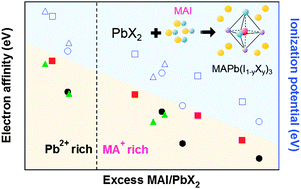
We fabricate mixed halide perovskite films through dual-source vacuum deposition of PbX2 (X = Cl, Br, and I) and methyl ammonium iodide (MAI) precursors with various deposition ratios. Vacuum deposition is an optimal way for film fabrication because it gives a uniform perovskite film which is free from contamination such as metallic phase lead, residual solvent, and moisture. The ionization potential and bandgap of MAPb(I1−yBry)3 film are controlled by changing the halide composition and lattice constant. In contrast, MAPb(I1−yCly)3 film shows negligible difference from MAPbI3 in terms of structural and electronic properties, which is due to poor Cl incorporation in the film from the MACl removal during crystal formation. An excess supply of MAI is necessary to form a perovskite crystal structure. Based on the elemental stoichiometry analysis, the additional methyl ammonium cation with respect to Pb in the film plays a critical role in changing the electron affinity and energy level alignment.

 Please wait while we load your content...
Please wait while we load your content...