Exploring the core level shift origin of sulfur and thiolates on Pd(111) surfaces
Abstract
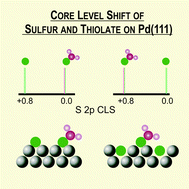
Thiol molecules on planar metal surfaces are widely used for building sensing and electronic devices and also as capping agents to protect and to control the size and shape of nanoparticles. In the case of Pd the thiol molecules exhibit a complex behavior because C–S bond scission is possible, resulting in a significant amount of co-adsorbed S. Therefore identification of these species on Pd is a key point for many applications, a task that is usually achieved by XPS. Here we show, from DFT calculations, that the core level shift (CLS) of the S 2p binding energy (BE) of thiol and sulfur on different thiol–Pd(111) surface models strongly depends on the adsorbed or subsurface state of sulfur atoms. Our results reflect the complexity of S 2p BE behavior and contribute to understanding and reanalyzing the experimental data of thiolated Pd surfaces.

 Please wait while we load your content...
Please wait while we load your content...