Structural and kinetic investigation of the hydride composite Ca(BH4)2 + MgH2 system doped with NbF5 for solid-state hydrogen storage†
Abstract
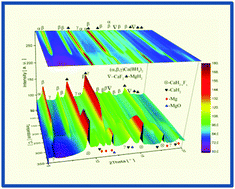
Designing safe, compact and high capacity hydrogen storage systems is the key step towards introducing a pollutant free hydrogen technology into a broad field of applications. Due to the chemical bonds of hydrogen–metal atoms, metal hydrides provide high energy density in safe hydrogen storage media. Reactive hydride composites (RHCs) are a promising class of high capacity solid state hydrogen storage systems. Ca(BH4)2 + MgH2 with a hydrogen content of 8.4 wt% is one of the most promising members of the RHCs. However, its relatively high desorption temperature of ∼350 °C is a major drawback to meeting the requirements for practical application. In this work, by using NbF5 as an additive, the dehydrogenation temperature of this RHC was significantly decreased. To elucidate the role of NbF5 in enhancing the desorption properties of the Ca(BH4)2 + MgH2 (Ca-RHC), a comprehensive investigation was carried out via manometric measurements, mass spectrometry, Differential Scanning Calorimetry (DSC), in situ Synchrotron Radiation-Powder X-ray Diffraction (SR-PXD), X-ray Absorption Spectroscopy (XAS), Anomalous Small-Angle X-ray Scattering (ASAXS), Scanning and Transmission Electron Microscopy (SEM, TEM) and Nuclear Magnetic Resonance (NMR) techniques.

 Please wait while we load your content...
Please wait while we load your content...