A systematic study of metal-supported boron nitride materials for the oxygen reduction reaction†
Abstract
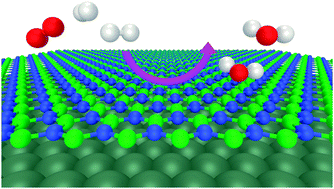
Surfaces that efficiently catalyse the oxygen reduction reaction (ORR) are highly desirable for applications in energy utilization. Here, we computationally investigate the ORR on hexagonal boron nitride (h-BN) supported on Ni, Cu, and Co. We find a significant influence of the metal on the reaction energetics. In particular, h-BN/Cu is predicted to catalyse the ORR with a low overpotential, while on the other substrates the reaction is impeded by the formation of too stable surface hydroxyl species. Our results highlight trends in the reactivity of these heterostructures and may guide further rational design of O2-activating catalysts based on supported h-BN.

 Please wait while we load your content...
Please wait while we load your content...