CO2 conversion to methanol on Cu(i) oxide nanolayers and clusters: an electronic structure insight into the reaction mechanism†
Abstract
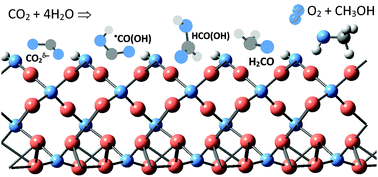
The mechanism of carbon dioxide reduction to methanol on Cu(I) oxide nanolayers and clusters using water as the source of hydrogen was traced using density functional theory. The nature of the active sites is revealed, namely the role of surface copper dimers, which are present on the Cu2O(001) surface and in the nanoclusters of size Cu32O16 and Cu14O7. The major difference between metal catalysts and Cu2O is outlined: the CO2 molecule interacts strongly with the oxide and undergoes bending prior to hydrogenation. The first step of CO2 hydrogenation results in the formation of a stable carboxyl intermediate, –CO(OH), which in the following steps is converted to methanol via formic acid and formaldehyde intermediates. The consumption of hydrogen from water leaves surface peroxo- and hydroperoxo-species. The peroxides easily desorb molecular oxygen, while for hydroperoxides the reaction of oxygen evolution requires an activation energy of 130 kJ mol−1. The maxima in the absorption spectra correspond well with the required activation energies in the elementary steps.

 Please wait while we load your content...
Please wait while we load your content...