Photoelectrochemical generation of hydrogen and electricity from hydrazine hydrate using BiVO4 electrodes†
Abstract
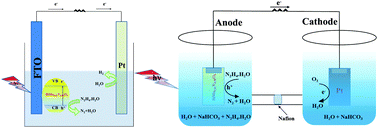
This study demonstrates solar driven oxidation of hydrazine hydrate and the simultaneous production of hydrogen and electricity in photoelectrochemical cells and photofuel cells, respectively, using a visible light active molybdenum doped BiVO4 photoelectrode. The developed photoelectrodes exhibited tremendous efficiency towards anodic oxidation of hydrous hydrazine with continuous and stable hydrogen evolution at the Pt cathode under benign pH and zero bias conditions. Significantly, the photofuel cell containing hydrazine hydrate fuel has generated electricity with a high open circuit potential of 0.8 V. The presence of bicarbonate ions in the electrolyte has played a significant role in enhancing the kinetics of photoelectrochemical oxidation of hydrazine and improved the hydrogen and electricity generation efficiency thus avoiding the integration of an oxidation electrocatalyst. In addition, molybdenum doped BiVO4 as a possible photoelectrochemical hydrazine sensor has been investigated and the electrode photocurrent was found to be linearly dependent on the concentration of the hydrazine hydrate in the range of 20–90 mM with a correlation coefficient of 0.9936.

 Please wait while we load your content...
Please wait while we load your content...