Ibuprofen causes photocleavage through ROS generation and intercalates with DNA: a combined biophysical and molecular docking approach
Abstract
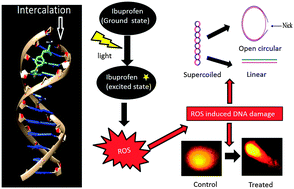
Ibuprofen is an important nonsteroidal anti-inflammatory drug endowed with various pharmacological and biological activities. In the present study, the photochemical properties of ibuprofen were evaluated by assaying the generation of various reactive oxygen species (ROS) such as superoxide, singlet oxygen and the hydroxyl radical. ROS generated by ibuprofen in the presence of white light causes DNA strand scission as observed by plasmid nicking assay. Ibuprofen induced ROS generation is also capable of causing DNA degradation in lymphocytes as observed by photocomet assay. ROS generation properties of ibuprofen were further strengthened by the formation of carbonyl groups in BSA and TBARS in linoleic acid as observed by carbonyl assay and lipid peroxidation assay respectively. We have also investigated the mode of interaction of ibuprofen with calf thymus DNA through a series of in vitro experiments. UV-visible spectroscopy established the formation of a complex between ibuprofen and Ct DNA. The steady state fluorescence experiments at different temperatures revealed a binding constant of ∼104 L mol−1, which is indicative of intercalative binding between ibuprofen and the DNA helix. Analysis of the various thermodynamic parameters ΔG, ΔH and ΔS calculated at different temperatures indicated that the hydrogen bonds played a major role in the interaction. The intercalative binding mode is further confirmed by competitive displacement assays, urea denaturation, iodide quenching, viscosity measurements and CD analysis. In silico molecular docking revealed the binding of ibuprofen within the GC base pairs of DNA, confirming the intercalative binding mode.

 Please wait while we load your content...
Please wait while we load your content...