Molecular features contributing to the lower viscosity of phosphonium ionic liquids compared to their ammonium analogues†
Abstract
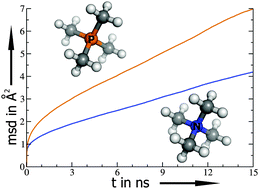
Molecular features contributing to the lower viscosity of phosphonium based ionic liquids (ILs) compared to ammonium based ILs are investigated by static quantum chemistry calculations and classical molecular dynamics simulations. The larger bond distance and the higher flexibility of bond angles and dihedral angles in the phosphonium compounds tend to reduce their viscosity compared to ammonium analogues, while the strongly localized charge at the central atom has the opposite effect. Fast translational ion dynamics is also found to be related to a short counter-ion association lifetime in the investigated compounds. Furthermore, a weak structuring between the center of charges also seems to increase mobility. Interestingly, the order of ion pair interaction energies in the gas phase is reversed compared to the order of counter-ion association lifetimes in the liquid, which highlights the important role of solvation in ILs. Overall, the higher flexibility of the bond and dihedral angles of the phosphonium compounds appears to be the most important factor in producing the lower viscosity of these ILs compared to their ammonium analogues.

 Please wait while we load your content...
Please wait while we load your content...