The working mechanism of the β-carbonic anhydrase degrading carbonyl sulphide (COSase): a theoretical study†
Abstract
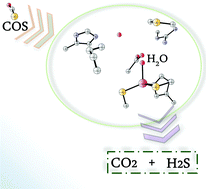
In order to give insights into the working mechanism of the novel characterized enzyme carbonyl sulphide hydrolase (COSase), which efficiently converts COS into H2S and CO2, we have performed a detailed theoretical investigation using the framework of density functional theory (using B3LYP and M06 exchange–correlation functionals) by the cluster model approach. In the final part of the reaction the metal ion is unable to form a pentacoordinated species. The B3LYP-D3 and M06 potential energy surfaces have a very similar shape. The elucidation of the catalytic reduction of COS is important in view of its role in environmental chemistry.

 Please wait while we load your content...
Please wait while we load your content...