Theoretical design of solvatochromism switching by photochromic reactions using donor–acceptor disubstituted diarylethene derivatives with oxidized thiophene rings†
Abstract
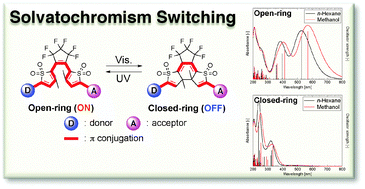
We have designed several diarylethene derivatives with oxidized thiophene rings and donor–acceptor substituents, which show the solvatochromism switching by photochromic reactions, using a time-dependent density functional theory (TD-DFT) method using the polarizable continuum model (PCM). It is found that in the UV-vis spectral region examined only the open-ring isomers exhibit the solvatochromism, while the closed-ring isomers do not. The mechanism of the solvatochromism behavior and its switching process are clarified from the viewpoint of the charge-transfer (CT) excitation from the donor to the acceptor substituents. We demonstrate that this CT excitation can be controlled by choosing appropriate pairs of the donor and the acceptor substituents on the basis of the orbital correlation diagram between the diarylethene derivatives and the donor–acceptor substituents, which is constructed from the topologies and the orbital energies of the molecular orbitals primarily contributing to the excitations.

 Please wait while we load your content...
Please wait while we load your content...