Containerless solidification of undercooled SrO–Al2O3 binary melts
Abstract
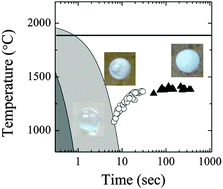
The solidification of the SrO–Al2O3 binary system was investigated under containerless conditions using an aerodynamic levitation furnace. Glass formation was observed in compositions with 35–45 mol% SrO and 55–75 mol% SrO. Cooling curves were obtained at a constant cooling rate in the range of 1–1000 °C s−1. The crystallization temperature was apparently independent of the cooling rate and far below the melting point when the sample was fully crystallized, whereas it decreased when the sample was partially crystallized. The difference between the crystallization temperature and the melting point under containerless conditions is considered a good measure of the glass-forming ability when there is not much difference in the critical cooling rates between the melt compositions. Furthermore, the homogeneous nucleation theory suggests that the apparent time-independent crystallization temperature is attributed to the high glass-forming ability of the SrO–Al2O3 binary system. The results suggest that the experimentally obtained continuous cooling transformation diagrams under containerless conditions provide new insights regarding solidification from an undercooled melt.

 Please wait while we load your content...
Please wait while we load your content...