Interplay between water uptake, ion interactions, and conductivity in an e-beam grafted poly(ethylene-co-tetrafluoroethylene) anion exchange membrane†
Abstract
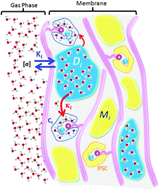
We demonstrate that the true hydroxide conductivity in an e-beam grafted poly(ethylene-co-tetrafluoroethylene) [ETFE] anion exchange membrane (AEM) is as high as 132 mS cm−1 at 80 °C and 95% RH, comparable to a proton exchange membrane, but with very much less water present in the film. To understand this behaviour we studied ion transport of hydroxide, carbonate, bicarbonate and chloride, as well as water uptake and distribution. Water uptake of the AEM in water vapor is an order of magnitude lower than when submerged in liquid water. In addition 19F pulse field gradient spin echo NMR indicates that there is little tortuosity in the ionic pathways through the film. A complete analysis of the IR spectrum of the AEM and the analyses of water absorption using FT-IR led to conclusion that the fluorinated backbone chains do not interact with water and that two types of water domains exist within the membrane. The reduction in conductivity was measured during exposure of the OH− form of the AEM to air at 95% RH and was seen to be much slower than the reaction of CO2 with OH− as the amount of water in the film determines its ionic conductivity and at relative wet RHs its re-organization is slow.

 Please wait while we load your content...
Please wait while we load your content...