Decomposition of fluorophosphoryl diazide: a joint experimental and theoretical study†
Abstract
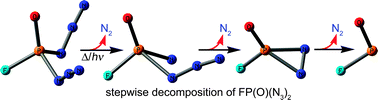
The photolytic and thermal decomposition of fluorophosphoryl diazide, FP(O)(N3)2, was studied using matrix isolation spectroscopy. Upon ArF laser photolysis (λ = 193 nm), FPO and a new geminal azido nitrene FP(O)(N3)N were identified using matrix IR spectroscopy. The nitrene shows a triplet ground state with the zero-field parameters |D/hc| = 1.566 cm−1 and |E/hc| = 0.005 cm−1. Further decomposition of the nitrene into FPO was observed under an irradiation of λ > 335 nm. In contrast, no nitrene but only FPO was identified after flash vacuum pyrolysis of the diazide. To reveal the decomposition mechanism, quantum chemical calculations on the potential energy surface (PES) of the diazide using DFT methods were performed. On the singlet PES four conformers of the nitrene were predicted. The two conformers (syn and anti) showing intramolecular Nnitrene⋯Nα,azide interactions are much lower in energy (ca. 40 kJ mol−1, B3LYP/6-311+G(3df)) than the other two exhibiting Nnitrene⋯O interactions. syn/anti refers to the relative orientation of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and the N3 group. The interconversion of these species and the decomposition into FPO via a novel three-membered ring diazo intermediate cyclo-FP(O)N2 were computationally explored. The calculated low dissociation barrier of 45 kJ mol−1 (B3LYP/6-311+G(3df)) of this cyclic intermediate rationalizes why it could not be detected in our experiments.
O bond and the N3 group. The interconversion of these species and the decomposition into FPO via a novel three-membered ring diazo intermediate cyclo-FP(O)N2 were computationally explored. The calculated low dissociation barrier of 45 kJ mol−1 (B3LYP/6-311+G(3df)) of this cyclic intermediate rationalizes why it could not be detected in our experiments.

 Please wait while we load your content...
Please wait while we load your content...