Atomistic simulations of ammonium-based protic ionic liquids: steric effects on structure, low frequency vibrational modes and electrical conductivity†
Abstract
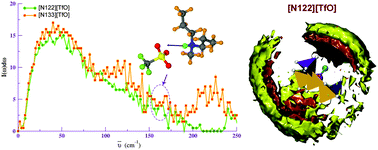
Protic ionic liquids (PILs) are of great interest as electrolytes in various energy applications. Molecular dynamics simulations of trialkylammonium (with varying alkyl group such as methyl, ethyl, and n-propyl) triflate PILs are performed to characterize the influence of the alkyl group on the acidic site (N–H) of the ammonium cation. Spatial distribution function of anions over this site on the cation reveals significant influence of the length of alkyl tail on intermolecular structure. Vibrational density of states and normal modes are calculated for bulk liquids to probe atomic displacements in the far infrared region. The observed N–H⋯O hydrogen bond stretching vibration in 155–165 cm−1 frequency region agrees well with experiments. Trends in electrical conductivity calculated using Nernst–Einstein and Green–Kubo relation are in qualitative agreement with experiments. The self-diffusion coefficient and the electrical conductivity is highest for N,N-dimethyl-N-ethylammonium triflate ([N112][TfO]) and is lowest for N,N-di-n-propyl-N-methylammonium triflate ([N133][TfO]) IL.

 Please wait while we load your content...
Please wait while we load your content...