Intra-residue interactions in proteins: interplay between serine or cysteine side chains and backbone conformations, revealed by laser spectroscopy of isolated model peptides
Abstract
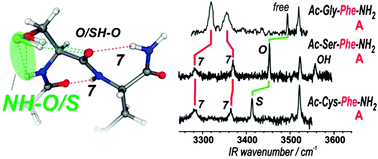
Intra-residue interactions play an important role in proteins by influencing local folding of the backbone. Taking advantage of the capability of gas phase experiments to provide relevant information on the intrinsic H-bonding pattern of isolated peptide chains, the intra-residue interactions of serine and cysteine residues, i.e., OH/SH⋯OC(i) C6 and NH(i)⋯O/S C5 interactions in Ser/Cys residues, are probed by laser spectroscopy of isolated peptides. The strength of these local side chain–main chain interactions, elegantly documented from their IR spectral features for well-defined conformations of the main chain, demonstrates that a subtle competition exists between the two types of intra-residue bond: the C6 H-bond is the major interaction with Ser, in contrast to Cys where C5 interaction takes over. The restricted number of conformers observed in the gas phase experiment with Ser compared to Cys (where both extended and folded forms are observed) also suggests a significant mediation role of these intra-residue interactions on the competition between the several main chain folding patterns.

 Please wait while we load your content...
Please wait while we load your content...