Capturing the structural diversification upon thermal desolvation of a robust metal organic framework via a single-crystal-to-single-crystal transformation†
Abstract
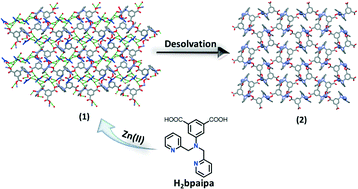
Using a new mixed polypyridyl–carboxylate ligand, a 2D neutral robust framework of Zn(II), {[Zn(bpaipa)]·DMF·2H2O}n (1) (where H2bpaipa = 5-(bis(pyridin-2-ylmethyl)amino)isophthalic acid), has been synthesized under solvothermal conditions in 71% yield. It undergoes single-crystal-to-single-crystal transformation upon thermal desolvation without a change in the topology of the framework where the desolvated structure shows an orientation change of an uncoordinated carboxylate oxygen atom and the pyridyl groups of the ligand within the pores. Both structures have been determined by single crystal X-ray analysis. Its high thermal stability (>350 °C) and framework integrity towards many solvents are also demonstrated by variable temperature powder X-ray diffraction and thermogravimetric analysis.
- This article is part of the themed collection: Single-Crystal-to-Single-Crystal Transformations

 Please wait while we load your content...
Please wait while we load your content...