Effect of fatty acid on the formation of ITO nanocrystals via one-pot pyrolysis reaction†
Abstract
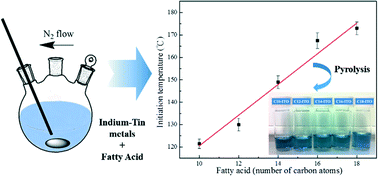
Indium–tin carboxylate precursors were successfully synthesized by a direct reaction of indium and tin metals with a molten fatty acid under a nitrogen atmosphere at 260 °C. A linear relationship between the reaction initiation temperature and the number of carbon atoms of the fatty acids ranging from capric acid to stearic acid was observed. There was a 7 °C increase in the reaction initiation temperature for an increase of one carbon atom in the fatty acid. Nearly monodisperse 7–9 nm ITO nanocrystals without agglomeration were synthesized by direct pyrolysis of the as-synthesized precursors without using additional organic solvents. The fatty acid had a minor effect on the decomposition temperature and the mean ITO particle size, but affected the particle size distribution. TOF-SIMS data confirmed that the residue fatty acids on the surface of the indium tin oxide (ITO) nanoparticles served as a built-in surfactant leading to excellent dispersity of the ITO nanocrystals in non-polar solvents.


 Please wait while we load your content...
Please wait while we load your content...