Synthesis and base pairing studies of geranylated 2-thiothymidine, a natural variant of thymidine†
Abstract
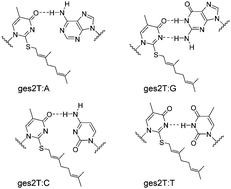
The synthesis and base pairing of DNA duplexes containing the geranylated 2-thiothymidine have been investigated. This naturally existing hydrophobic modification could grant better base pairing stability to the T–G pair over normal T–A and other mismatched pairs in the duplex context. This study provides a potential explanation for the different codon recognition preferences of the geranylated tRNAs.

 Please wait while we load your content...
Please wait while we load your content...