Stability and its manifestation in the chemical and biological worlds
Abstract
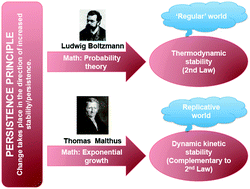
Bridging between the phenomenologically distinct biological and physical worlds has been a major scientific challenge since Boltzmann's probabilistic formulation of the second law of thermodynamics. In this review we summarize our recent theoretical attempts to bridge that divide through analysis of the thermodynamic-kinetic interplay in chemical processes and the manner in which that interplay impacts on material stability. Key findings are that the term ‘stability’ manifests two facets – time and energy – and that stability's time facet, expressed as persistence, is more general than its energy facet. That idea, together with the proposed existence of a logical law of nature, the persistence principle, leads to the mathematically-based insight that stability can come about through either Boltzmann's probabilistic considerations or Malthusian kinetics. Two mathematically-based forms of material persistence then lead directly to the physical likelihood of two material forms, animate and inanimate. Significantly, the incorporation of kinetic considerations into the stability concept appears to bring us closer to enabling two of the central theories in science – the second law of thermodynamics and Darwin's theory of evolution – to be reconciled within a single conceptual framework.


 Please wait while we load your content...
Please wait while we load your content...