A general method for N-glycosylation of nucleobases promoted by (p-Tol)2SO/Tf2O with thioglycoside as donor†
Abstract
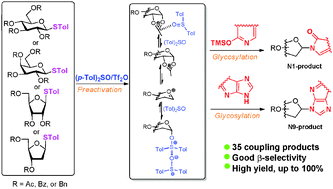
Based on a preactivation strategy using the (p-Tol)2SO/Tf2O system, a series of nucleosides were synthesized by coupling various thioglycosides with pyrimidines and purines under mild conditions. High yields and excellent β-stereoselectivities were obtained with either armed or disarmed N-glycosylation donors by tuning the amount of (p-Tol)2SO additive.

 Please wait while we load your content...
Please wait while we load your content...