Oxidation of carbon monoxide in basic solution catalyzed by nickel cyano carbonyls under ambient conditions and the prototype of a CO-powered alkaline fuel cell†
Abstract
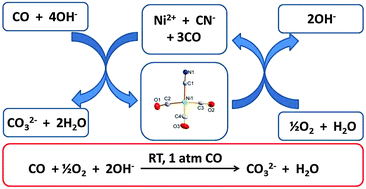
CO reduces nickel(2+) cyanides in basic solution to form tetrahedral Ni0(CN)(CO)3− or Ni0(CN)2(CO)22−. Both nickel(0) complexes can be oxidized back to nickel(2+) cyanide/hydroxide so that they behave as CO oxidation catalysts in basic solution. A primitive fuel cell was constructed to demonstrate the feasibility of a CO-powered fuel cell.

 Please wait while we load your content...
Please wait while we load your content...