Regioselective C–H bond amination by aminoiodanes†
Abstract
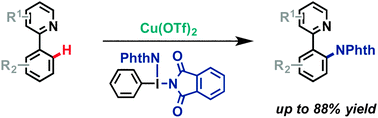
A new approach for the direct amination of 2-phenylpyridine derivatives using a diphthalimide-iodane and copper triflate has been developed. A series of different 2-phenylpyridine derivatives were aminated with yields up to 88%. Mechanistic investigations indicate that the reaction proceeds via a copper-mediated single electron transfer.

 Please wait while we load your content...
Please wait while we load your content...