Iron oxide cluster induced barrier-free conversion of nitric oxide to ammonia†
Abstract
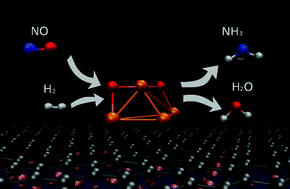
Nitrogen oxide (NO) conversion to ammonia (NH3) over iron oxide clusters is investigated using density functional theory calculations. The introduction of NO and H2 over gas-phase Fe4O2 results in the formation of NH3 and H2O without activation barriers. The key reaction is barrier free conversion of NO to N and H2O where the physical origin rests on negative–negative repulsion of NO over iron oxide clusters. The by-product H2O can be a hydrogen source due to electrolysis or re-visiting Fe4O2 and hence, the reaction is self-sustainable. Furthermore, the above reactions are performed by depositing Fe4O2 clusters on graphene/Cu(111) where the barrier free conversion of NO to NH3 is also observed, predicting that such reaction can be applicable. Thus, low temperature conversion of NO to NH3 over Fe4O2 can be predicted. The detailed reaction pathway and mechanism are presented.


 Please wait while we load your content...
Please wait while we load your content...