Synthesis, structure and dehydrogenation of zirconium borohydride octaammoniate†
Abstract
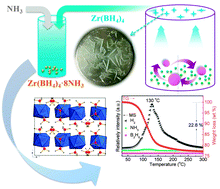
A new metal borohydride ammoniate (MBA), Zr(BH4)4·8NH3, was synthesized via ammoniation of the Zr(BH4)4 crystal. Zr(BH4)4·8NH3 has a distinctive structure and the highest coordination number of NH3 groups among all the known MBAs. This compound could quickly dehydrogenate at 130 °C, enabling it a potential hydrogen storage material.

 Please wait while we load your content...
Please wait while we load your content...