Highly selective synthesis of functionalized polyhydroisoquinoline derivatives via a three-component domino reaction†
Abstract
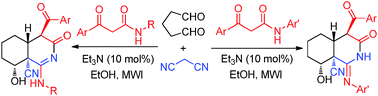
Two series of novel functionalized polyhydroisoquinoline derivatives have been synthesized via the three-component domino reaction of glutaraldehyde and malononitrile with a series of β-ketoamides under microwave irradiation conditions in the presence of a catalytic amount of Et3N (10 mol%). This reaction represents the first reported process for the facile conversion of a β-ketoamide to a hydroisoquinoline via a C–N bond cleavage reaction without the need for a multistep reaction process.

 Please wait while we load your content...
Please wait while we load your content...