A robust and modular synthesis of ynamides†
Abstract
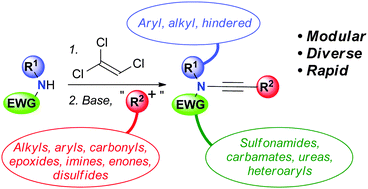
A flexible, modular ynamide synthesis is reported that uses trichloroethene as an inexpensive two carbon synthon. A wide range of amides and electrophiles can be converted to the corresponding ynamides, importantly including acyclic carbamates, hindered amides, and aryl amides. This method thus overcomes many of the limitations of other approaches to this useful functionality.

 Please wait while we load your content...
Please wait while we load your content...