A cyanine-derived “turn-on” fluorescent probe for imaging nitroreductase in hypoxic tumor cells†
Abstract
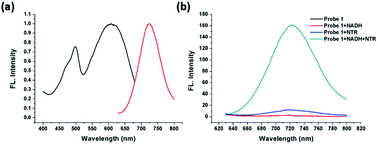
A new “turn-on” fluorescent probe, composed of a protected phenol group with a p-nitrobenzyl moiety that functions as a latent donor and conjugated with two benzo[f]indolinium acceptors, was developed and applied for imaging nitroreductase (NTR) in hypoxic tumor cells. Nitrobenzyl moieties on the substrate can be conveniently converted into aminobenzyl groups with a nitroreductase-catalyzed reaction in the presence of reduced nicotinamide adenine dinucleotide (NADH). This is followed by a cleavage reaction and the release of the free phenol moiety, which is manifested in enhanced fluorescence intensity during the detection process. Our experimental results show that the NTR detection ability is over 100 equivalents of other biological reductants (1 mM), whereas the confocal fluorescence imaging of tumor cells indicates the possibility of its application in biomedical research fields for tumor hypoxia diagnosis.

 Please wait while we load your content...
Please wait while we load your content...