Selective recognition and sensing for adenosine triphosphate by label-free electrochemistry based on its inclusion with per-6-ammonium-β-cyclodextrin†
Abstract
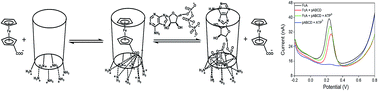
A novel method for the selective sensing of ATP was developed using ferrocenecarboxylic acid (FcA) as an electrochemical probe, based on the competitive reaction between ATP (or ADP, AMP) and FcA with per-6-ammonium-β-cyclodextrin (pABCD). pABCD was synthesized in four steps from β-cyclodextrin by an improved literature method. In the pABCD molecule, the primary hydroxyl groups at position 6 of β-cyclodextrin have been substituted by amino groups. The presence of amino groups increased the binding ability. pABCD showed the strongest binding ability towards adenosine triphosphate (ATP), not only due to the host–guest inclusion of the cavity of pABCD with the adenosine base, but also the interaction of the positively charged ammonium groups of pABCD with the phosphate anion moieties. FcA, as an excellent electroactive probe, can be included in the pABCD cavity and produce a decreased oxidation signal. However, when ATP was added to the pABCD–FcA system, the oxidation peak current increased with increasing concentrations of ATP. In this way, the pABCD–FcA system can recognize and sense ATP through the competitive interaction of ATP and FcA with pABCD. The increased signal of FcA upon the addition of ATP into the FcA–ABCD system was studied using differential pulsed voltammetry (DPV). The inclusion constants of pABCD with FcA, ATP, ADP and AMP were evaluated using DPV by application of the Langmuir equation 1/ΔI = 1/ΔImax + 1/(kcΔImax). Based on these results, under the optimized experimental conditions, the oxidation current of FcA in the FcA–pABCD system responding to the concentration of ATP was linear in the range of 3.12 × 10−7 to 1.68 × 10−6 mol L−1, with a correlation coefficient of 0.9978 and a detection limit (S/N = 3) of 1.43 × 10−7 mol L−1. These results demonstrate that this method is highly selective and sensitive for the determination of ATP.

 Please wait while we load your content...
Please wait while we load your content...