An efficient colorimetric and fluorescent probe for the detection of fluoride ions based on a benzothiadiazole derivative†
Abstract
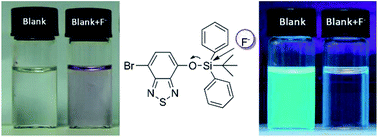
In this paper, an efficient colorimetric and fluorescent probe based on a benzothiadiazole derivative was developed for the detection of fluoride ions (F−). The selectivity of this approach capitalized on the highly selective and straightforward chemical reaction between F− and the Si–O bond. The sensing behavior of this probe towards anions was monitored by UV-visible and fluorescence spectroscopies in acetonitrile–aqueous buffer mixtures. Among the tested anions: F−, Cl−, Br−, I−, ClO4−, NO3−, CN−, HSO4−, AcO− and H2PO4− only F− induced a remarkable red-shift (149 nm) in the absorption spectrum which is concomitant with a color change from colorless to pink. This probe provides a new approach for naked-eyed detection of F− and the detection limit was calculated to be 1.7 μM. Besides, a concomitant fluorescence decrease was also observed. These results demonstrated that the probe could detect F− with excellent sensitivity and selectivity in complicated samples.

 Please wait while we load your content...
Please wait while we load your content...