Ion-induced cycle opening of a diarylethene and its application on visual detection of Cu2+ and Hg2+ and keypad lock†
Abstract
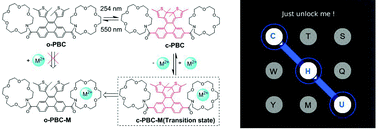
A phenanthrene-bridged photochromic diarylethene with two crown ethers as ion recognizing groups has been synthesized and characterized by single crystal X-ray diffraction. This material shows good photochromism and fatigue resistance. Only the closed form of this diarylethene derivative can form coordination complexes with Cu2+ and Hg2+ and at the same time, the combination of the photochromic material and metal ions would lead to the cycle opening of the closed photochromic unit and color bleaching processes. This special property can be applied in the visual detection of these two heavy metal ions not only in solution but also in solid state. The fluorescence of the diarylethene is sensitive to light, copper and mercury ions. As a result, a single molecular logic circuit was constructed using the absorbance and fluorescence intensity at a specific wavelength as outputs and appropriate combinational stimuli of UV/Vis light, Cu2+ and Hg2+ ions as inputs. Moreover, its fluorescence is sensitive to historical input signals. Based on this, a key pad lock using three codes to open was fabricated.

 Please wait while we load your content...
Please wait while we load your content...