Highly activated K-doped iron carbide nanocatalysts designed by computational simulation for Fischer–Tropsch synthesis†
Abstract
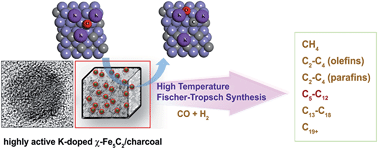
Although the reaction results of numerous iron-based Fischer–Tropsch synthesis catalysts containing various promoters have been reported, the research on their theoretical foundation is still insufficient. In the present work, highly activated K-doped χ-Fe5C2/charcoal nanocatalysts were designed using calculations based on density functional theory (DFT), and then prepared using a melt-infiltration process and a subsequent incipient-wetness method of K precursors. The catalyst at K/Fe = 0.075 in an atomic ratio that bears small iron carbide nanoparticles of ∼18 nm showed the highest activity (1.54 × 10−4 molCO gFe−1 s−1) and the best hydrocarbon yield (1.41 × 10−3 gHC gFe−1 s−1), as well as a good selectivity for gasoline-range (C5–C12) hydrocarbon products in the high-temperature Fischer–Tropsch reaction.

 Please wait while we load your content...
Please wait while we load your content...