Zeolite growth by synergy between solution-mediated and solid-phase transformations†
Abstract
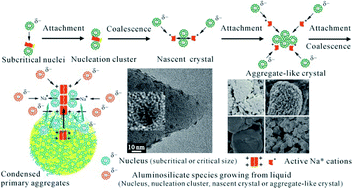
Understanding of the crystal growth mechanism of zeolites is essential for rational design of zeolite materials with desired physical and chemical properties, but still remains elusive. This paper describes experimental findings of zeolite crystal evolution from sodium-rich hydrogels, revealing that the zeolite nucleation occurs at the equilibrated gel phase of the condensed primary aggregates precipitated from the dissolved (alumino)silicate species. The nuclei produced from the nucleation could be diffused into the liquid–solid interface of the equilibrated gel phase and the liquid phase. The zeolite growth therefore occurs through a synergistic mechanism of two growth processes: a solution-mediated process and a solid-state transformation. In the liquid phase and the liquid–gel (equilibrated gel) interface, the oriented aggregation governs the zeolite growth in early stages. The major driving force for the aggregation is the electrostatic force between the positively charged active Na+ and the negative charges of the (TO−) groups on the surface of the nuclei and growing nanocrystals. In the last few steps the crystal growth by the coalescence and the Ostwald rule becomes predominant.

 Please wait while we load your content...
Please wait while we load your content...