Formal hydrogenation of arynes with silyl Cβ–H bonds as an active hydride source†
Abstract
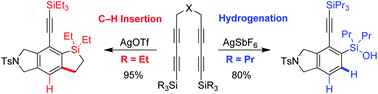
Formal hydrogenation of arynes was realized by using trialkylsilyl groups tethered to the arynes as the hydride source. In stark contrast to the effective 1°, 2°, and 3° C–H bond insertion of alkyl groups tethered to arynes, the 2° and 3° C–H bonds on the β-carbon of silyl groups show high tendency for hydride transfer rather than C–H insertion, whereas the corresponding 1° C–H bonds exclusively undergo C–H insertion. The exclusive hydride transfer and C–H insertion behavior of different C–H bonds in these two reaction pathways were rationalized by the stability of the incipient carbocationic intermediates which could be further bolstered by DFT-calculations.

 Please wait while we load your content...
Please wait while we load your content...