Light alkane oxidation using catalysts prepared by chemical vapour impregnation: tuning alcohol selectivity through catalyst pre-treatment†
Abstract
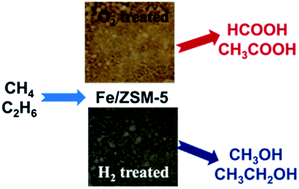
Fe/ZSM-5(30) catalysts have been prepared by chemical vapour impregnation (CVI) using iron(III) acetylacetonate as the precursor. These materials have been used for the oxidation of methane and ethane using aqueous hydrogen peroxide as oxidant. Heating in air leads to materials that exhibit high catalytic activity and give formic and acetic acid with high selectivity from methane and ethane respectively. Heat treatment of the uncalcined materials under a reducing atmosphere results in partial reduction of iron from the FeIII to FeII oxidation state with the majority of the iron being present as isolated octahedral extra-framework species having oxygen neighbours and showing no evidence of a coordination shell containing Al or Fe, as evidenced from studies using X-ray absorption and UV-Vis spectroscopies. These hydrogen treated catalysts show the same catalytic activity as their analogues formed by heating in air, but in contrast exhibit higher alcohol selectivities for both methane and ethane conversion to oxygenates and are reusable. Our findings for both the oxidation of methane and ethane indicate that the selectivity to the oxidation products, i.e. acids or alcohols, can be controlled by tuning the active site structure and/or oxidation state of the Fe species in Fe/ZSM-5.

 Please wait while we load your content...
Please wait while we load your content...