Enhancing the electro catalytic activity of manganese ferrite through cerium substitution for oxygen evolution in KOH solutions
Abstract
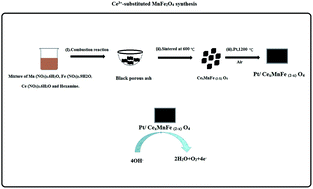
Alkaline water electrolysis (AWE) is the simplest way of producing hydrogen, an attractive fuel for the future. In view of their cost-effectiveness and durability, non-noble metal oxides are promising catalysts for AWE. Here, we studied the effect of Ce substitution on the OER activity of manganese ferrite. Ce substituted MnFe2O4 (0 ≤ x ≤ 0.8) was synthesized by a combustion method. Characterization techniques such as SEM, XRD, EDAX and XPS were used to analyse the surface morphology and the chemical composition of CexMnFe2−xO4. Substitution of Ce3+ in the cubic lattice of MnFe2O4 increases the conductivity of CexMnFe2−xO4, which results in the negative shift in the OER onset potential. Among all the Ce3+ substituted manganese ferrites, Ce0.2MnFe1.8O4 was found to be more active for OER in terms of current and stability. Notably, Ce0.2MnFe1.8O4 affords a current density of 10 mA cm−2 at a small overpotential of ∼310 mV and a Tafel slope value of 31 mV per decade, these values are comparable to the well-investigated non-noble metal oxides.

 Please wait while we load your content...
Please wait while we load your content...