Terpenes in honey: occurrence, origin and their role as chemical biomarkers
Abstract
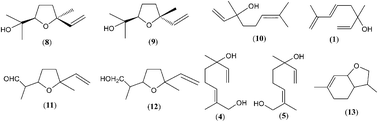
The major developments and concepts in the study of terpenes (mainly monoterpenes) as well as C9-, C10-, C13-, C14- and C15- norisoprenoids in honey are summarized. Their occurrence and biosynthetic correlations (terpene transformations, particularly the generation of linalool derivatives as well as carotenoid degradation and the corresponding variety of norisoprenoids) are discussed considering the plant/nectar/bee-stomach composition and hive conditions (e.g. warm and acidic conditions that can lead to oxidative degradation of compounds). Terpenes up to C15 have been found as major compounds of the essential oils, but honeys from the same plant sources exhibit only partial similarity to the oil composition (e.g. lavender, sage or citrus essential oils/honeys). The formation of heat-derived or prolonged storage artefacts in honey (mainly the products of Maillard reactions and Strecker degradation reactions) influence the honey composition, and hotrienol is particularly labile among the chemical groups discussed. Stimulated by the challenges of fingerprinting methods and structure identification, exploratory studies (including our published results) on terpenes and norisoprenoids are summarized with emphasis on identifying specific or nonspecific chemical markers of the botanical origin of honey. In general, nonspecific biomarkers dominate in different honey types (e.g. monoterpenes: linalool, linalool oxide isomers or lilac alcohol/aldehyde isomers; norisoprenoids: isophorone and vomifoliol derivatives) while only a few specific biomarkers are found (e.g. anhydrolinalool oxide isomers, 3,4-dihydro-3-oxoedulan isomers, 3-oxo-retro-α-ionol isomers, kamahine A-C or meliracemoic acid).

 Please wait while we load your content...
Please wait while we load your content...